MACI surgery offers healthcare providers the versatility to treat cartilage defects in multiple areas, including the femoral condyles, patella and trochlea. A recent case explores how a 23-year-old former college athlete pursued surgical treatment to address knee cartilage pain caused by a trochlear defect. Review the patient’s case history, treatment with MACI knee cartilage surgery and outcome.
The Patient
- 23-year-old male patient
- Former college lacrosse player, plays recreational basketball and snowboards
Patient’s Knee History
- Recurrent damage from continued athletics and small injuries
- Pain worsening over last two years.
- Swelling occurred with kneeling, pain and popping with any squatting or cutting
- No prior surgery
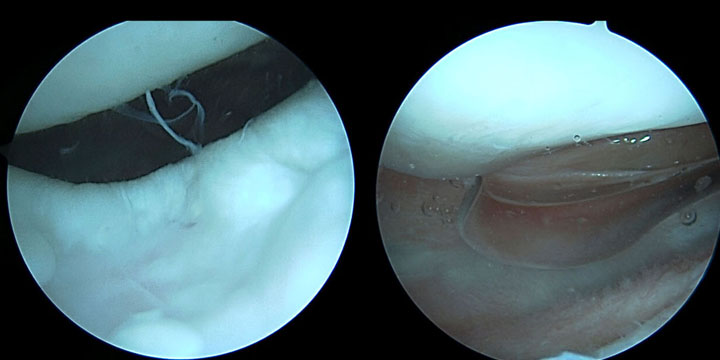
Arthroscopy Findings
- Arthroscopy revealed a trochlear defect of 2.5 x 2 cm
- Meniscus and patella both intact
- No bony involvement, good surrounding cartilage
- No comorbidities
Surgical Approach
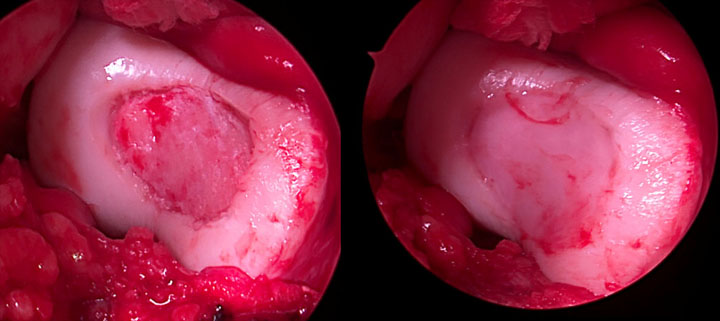
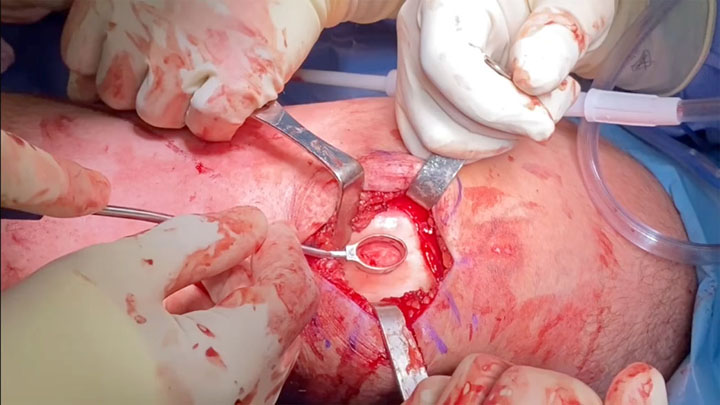
Following the initial arthroscopy, Dr. Garcia proceeded with MACI surgery and took a multi-pronged approach:
- Due to the size of the defect, a larger cartilage procedure was warranted, so MACI surgery was selected
- The defect size post debridement equaled 2.5 x 2 cm and one MACI implant was used during the surgery
Rehab
- Brace for 6 weeks, continuous passive motion machine (CPM) x 3 weeks, advance diet as tolerated (ADAT)
- Weight-bearing-as tolerated (WBAT) when straight leg raise test performed
- Goal range of motion 0–90 after first 2 weeks
- Stairs avoided for 6 months, no squats first 12 weeks, then progressed to full squat
- Started elliptical & bike 3 months
- Started running around 6 months
Outcome
- Patient returned to all sports at 9 months post-op with full range of motion and no swelling
Explore MACI knee cartilage repair clinical trials and patient outcomes here.
Individual results may vary.
Please see below for full indication and ISI. Blog posts are intended to provide educational information only and do not constitute medical advice.

