MACI knee cartilage repair treats symptomatic, full-thickness cartilage defects of the adult knee and is an option to treat patients with multiple lesions. Review a recent case study documenting treatment with MACI surgery and concomitant tibial tubercle osteotomy (TTO) for a 19-year-old collegiate athlete with defects of the lateral trochlea and patella.
The Patient
- 19-year-old female patient
- Active collegiate pole vaulter
- Presents with knee pain, swelling and mechanical symptoms
- Eighteen months prior to presentation, she underwent a left knee arthroscopy with loose body removal and chondroplasty. Patient recovered well post-op and returned to competition
Patient’s Knee History
- Two months prior to presentation, patient landed awkwardly from a vault. She experienced immediate pain in her left knee and subsequent swelling
- Initially treated injury with ice and NSAIDs but only provided minimal improvement
- Minimal to no underlying bone marrow edema
- Physician participated in a long discussion with patient, parents, and trainer regarding treatment options including platelet-rich plasma (PRP) and bone marrow aspirate (BMA) injections
- An examination uncovered the following in the patient’s left knee:
- Mild to moderate effusion
- Tenderness about the lateral patellar facet and anterolaterally over the lateral trochlea
- No medial or lateral joint line tenderness
- Range of motion: 0–125 degrees with pain on end flexion
- Repetitive painful catch between 20 and 30 degrees of knee flexion
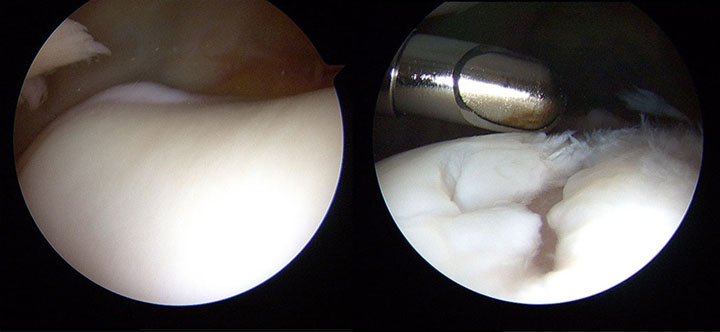
Surgical Approach
- Dr. Eric Strauss moved forward with MACI knee cartilage repair surgery to repair the defects:
- Arthroscopy revealed lateral trochlear defect measuring 10 x 12 mm and a patellar facet defect measuring 16 x 18 mm
- Cell-based cartilage repair via MACI to both sites with an unloading tibial tubercle osteotomy (60-degree cut)
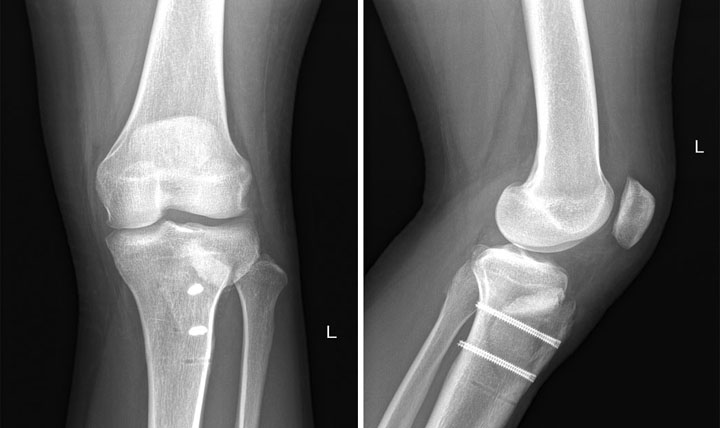
Rehab
- Dr. Strauss prescribed an 8-week rehab protocol with an anticipated return to low-impact activity in months 6 to 9
- Patient followed MACI rehab protocol under the guidance of a physical therapist and athletic trainer
- Used crutches for 6 weeks
- Began using the stationary bike at 8 weeks, elliptical at 4 months, and jogging at 9 months
- During 2 months of post-operative rehabilitation:
- Mechanical symptoms improved but still with pain and swelling episodes
- Patient tried to train/vault but was unsuccessful, secondary to pain and limited range of motion
Outcome
- At 9 months post-op, patient denies any operative site pain, swelling, or mechanical symptoms
- Range of motion: 0–140 degrees without pain
- Strength and endurance in quadriceps improving
- Returned to training and competition at 1-year post-op
- The season following surgery, she accomplished a personal best vault of 4.10 m
Explore more knee cartilage defects that may be treatable with MACI surgery here.
Individual results may vary.
Please see below for full indication and ISI. Blog posts are intended to provide educational information only and do not constitute medical advice.

