Research finds “time matters” when it comes to delays between patient biopsy and MACI implantation surgery.
The study, “Time Matters: Knee Cartilage Defect Expansion and High-Grade Lesion Formation while Awaiting Autologous Chondrocyte Implantation” by Dr. David Flanigan and a team of researchers, shows that patients undergoing cell-based surgery experienced new cartilage defects and progression in existing cartilage defects during delays between cartilage biopsy and chondrocyte implantation.
The Study Findings
The study examined consecutive knee ACI and MACI cases by a single surgeon (128 knees in 111 patients).
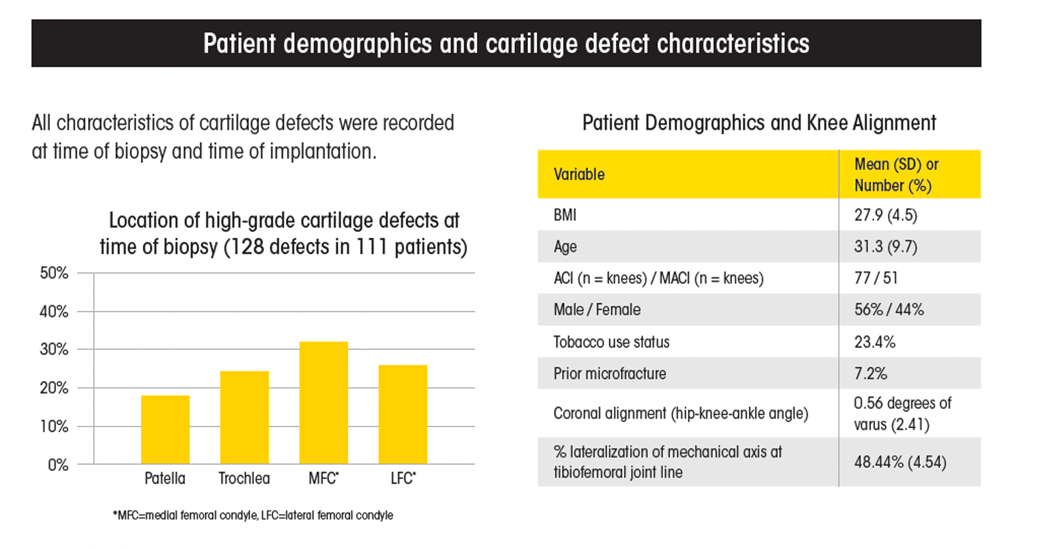
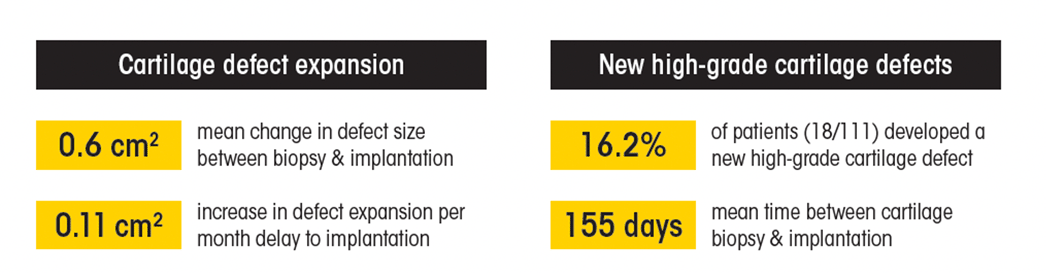
The mean time between cartilage biopsy and implantation was 155 days. In that time, patients’ cartilage defects expanded a mean of 0.6 cm2. Further, 16.2 percent of patients experienced new high-grade cartilage defects.
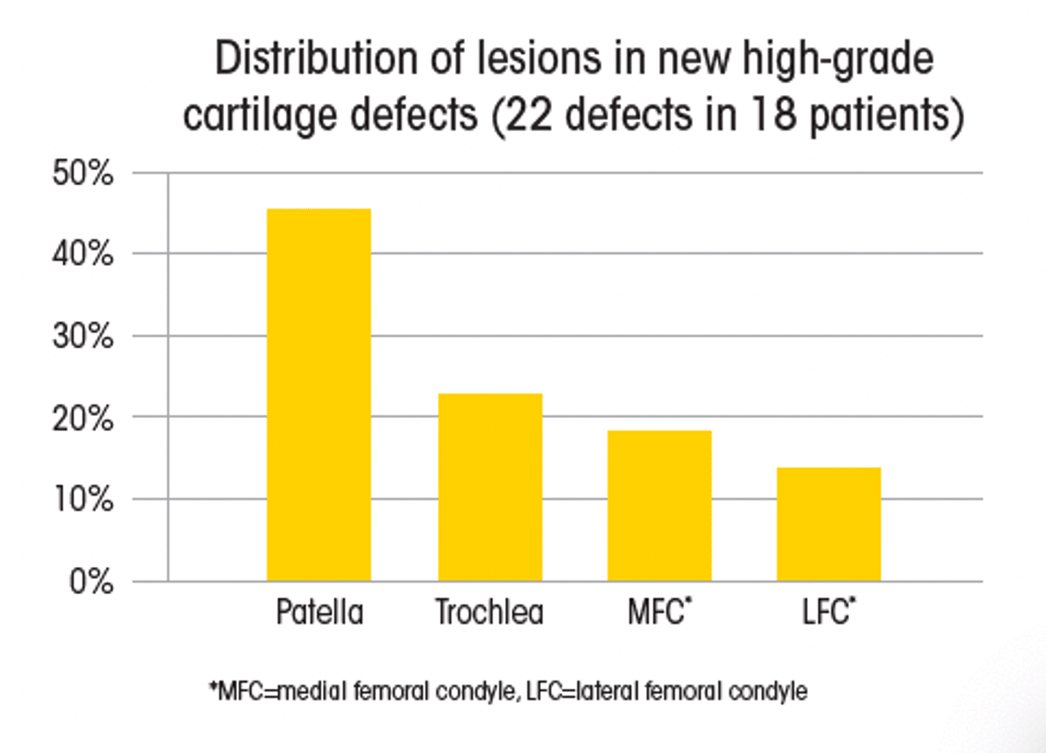
Of those patients who developed a new high-grade cartilage defect, the most common new defect location was the patella.
Who’s at Greatest Risk?
The study found that patients who were male, had smaller initial defect sizes, and longer time between surgeries were at greater risk for defect expansion.

Why Do Delays Occur?
Delays between biopsy and implantation can be affected by several variables, including chondrocyte processing, insurance approval, patient factors (preference for timing, deductibles, life events, etc.) and surgeon scheduling.
How Should you Advise Patients on Surgery Schedules?
Surgeons, patients, insurance, and industry should coordinate efforts to expedite the time between biopsy and implantation to prevent further deterioration of the knee joint and obtain better patient outcomes, the study authors wrote.
Please see below for full indication and ISI.

