The first step in treating knee cartilage damage via MACI is an arthroscopy. The knee arthroscopy allows a physician to scope the knee and harvest a biopsy of a patient’s cartilage cells, which are later used to generate a MACI knee cartilage implant.
Since ACI’s inception in 1987, surgeons have developed tried and true techniques for the initial arthroscopy and biopsy collection.
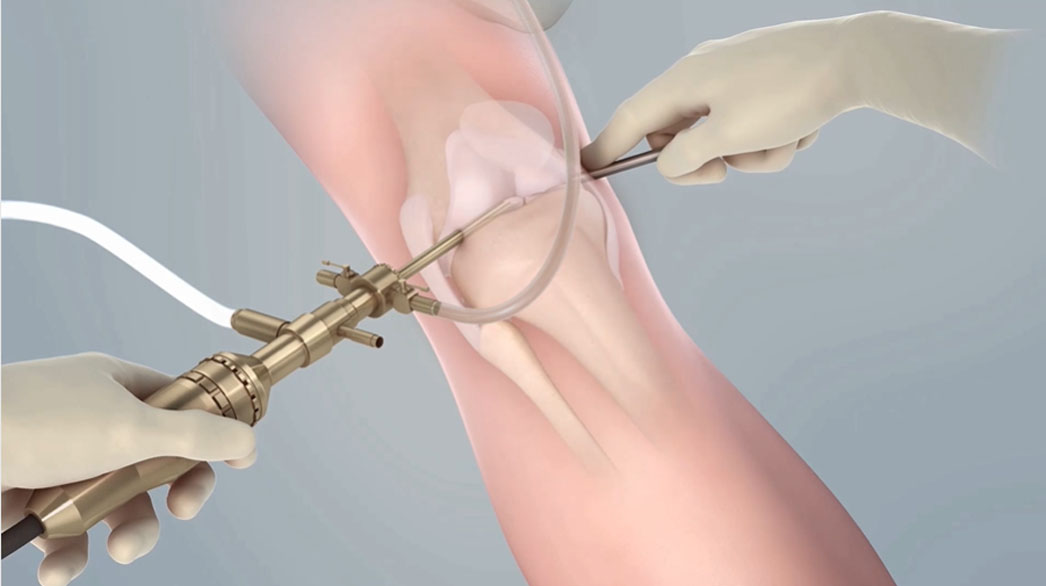
Review our three-part online seminar series “Work of Arthroscopy” for guidance on optimizing the procedure for positive patient outcomes.
The following practices do not constitute medical advice, and patients should be assessed according to their unique circumstances.
Session 1: Why Staged Treatment of Cartilage is Right for Your Patient?

Get an intro to MACI knee cartilage repair hosted by Dr. Paul Caldwell. It will cover:
- Insight into the MACI process and its potential benefits
- How to talk to patients about cartilage treatment options without causing fear
- Information on the MACI insurance approval process
- A profile of the patient who may consider MACI knee cartilage repair
REQUEST A WEBINAR – Session 1: Art of Arthroscopy
Session 2: Find Guidance for Intra-Articular Knee Evaluation and Biopsy
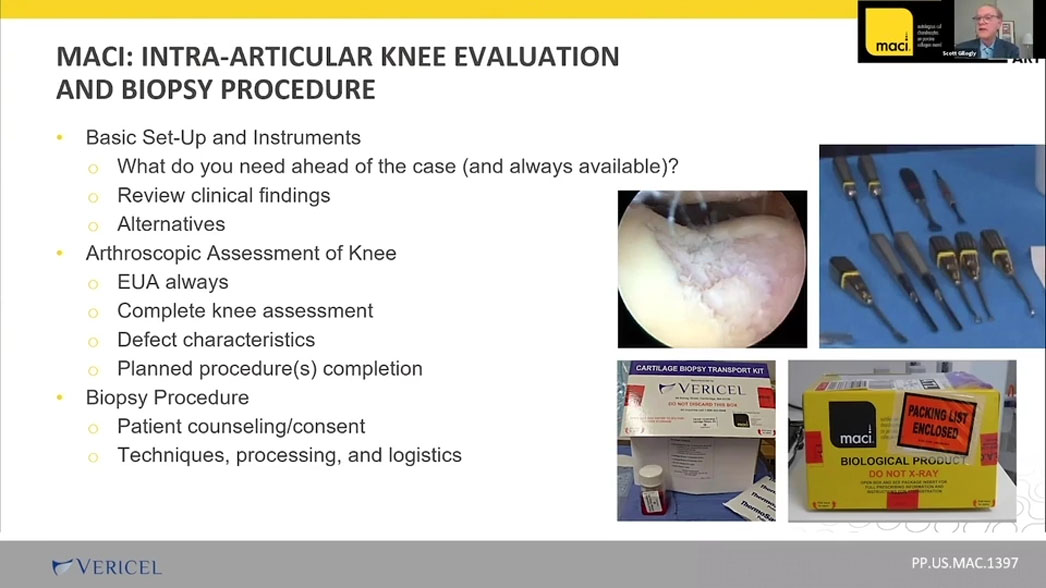
Dr. Scott Gillogly walks us through best practices for the arthroscopy procedure including:
- Basic instruments and set up of the arthroscopy
- His approach to the arthroscopic survey of the knee
- Criteria to identify knee cartilage lesions appropriate for MACI
- Appropriate biopsy harvest sites
- Cartilage sample harvest techniques using arthroscopic instruments such as a ring curette and a notchplasty gouge
REQUEST A WEBINAR – Session 2: Art of Arthroscopy
Session 3: The “What Ifs”: Considerations in the Planning of Articular Cartilage Restoration

Dr. Wayne Gersoff covers various complicating knee pathologies physicians encounter during an arthroscopy and how you can address them. Learn your options for scenarios including:
- Patellofemoral instability
- ACL deficiency
- Bone deficiency
- Meniscal injury
- Bone marrow edema
- Altered knee environment
REQUEST A WEBINAR – Session 3: Art of Arthroscopy
Please see below for full indication and ISI.

