MACI is the third generation of autologous chondrocyte implantation and has made multiple advancements that allow for greater flexibility in treating symptomatic knee cartilage defects in the adult knee.
Effectiveness of MACI in joints other than the knee has not been established. Safety and effectiveness of MACI in patients over the age of 55 years have not been established.

Previous ACIs were only approved for the treatment of the tibiofemoral joints. Because of the versatility offered by the MACI membrane during implantation, surgeons can now treat multiple areas of the knee, including more difficult to treat cartilage damage, like uncontained defects.
MACI is an appropriate option for the defects below:
- Lateral femoral condyle
- Medial femoral condyle
- Patella
- Trochlea
While MACI can be used for any size defect, it is typically used for full-thickness cartilage defects of the knee. More than 50% of MACI patients are treated for a condyle defect ranging in size from 2 cm2 to 8 cm2.
Other factors to consider when recommending MACI:
Patient Age
- MACI cartilage treatment is approved for patients 18–55. It has not been studied outside of that age range.
Symptoms
- MACI may be indicated in patients complaining of symptoms including sharp or dull pain in the front of the knee, bone on bone knee pain, pain and stiffness after prolonged sitting ("moviegoers' knee"), intermittent swelling, crepitus, and knee locking or catching.
Getting Patients Back to Activities They Love
- Has your patient struggled to maintain an active lifestyle because of knee cartilage damage? The patient may be struggling to participate in athletics or even everyday activities, like going down the stairs. MACI can help these knee pain sufferers regain their active lives.
Rehabilitation Timeline
- Recovery from MACI surgery is highly individualized and generally occurs over nine plus months. The rehabilitation program is tailored specifically to each patient's unique goals and objectives. MACI is contraindicated in patients who are unable to follow a physician-prescribed post-surgical rehabilitation program.
- The most frequently occurring adverse reactions reported for MACI (≥5%) were arthralgia, tendonitis, back pain, joint swelling, and joint effusion. Serious adverse reactions reported for MACI were arthralgia, cartilage injury, meniscus injury, treatment failure, and osteoarthritis. Please see full ISI and link to PI below.
The following patient profiles, based on actual case studies, may help you identify similar patients in your practice who may be eligible MACI candidates:
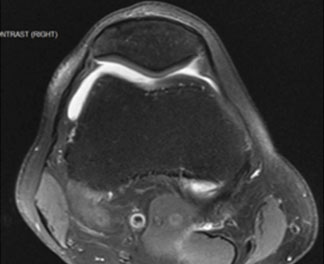 Patient 1 has a single 9 cm2 cartilage lesion with boney involvement in the left medial femoral condyle.
Patient 1 has a single 9 cm2 cartilage lesion with boney involvement in the left medial femoral condyle.
- Age: 52
- Gender: Male
- Occupation: Self-employed
- Hobbies: Enjoys hiking and tennis
- Symptoms: Recurrent knee pain from a hiking fall two years ago
- Prior treatments: None
- Imaging: Revealed full-thickness cartilage defects on MFC and indicated moderate compartment narrowing with no malalignment
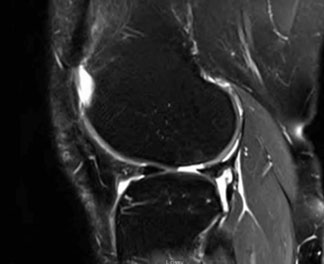 Patient 2 has a 2.2 cm2 cartilage lesion in the right lateral femoral condyle.
Patient 2 has a 2.2 cm2 cartilage lesion in the right lateral femoral condyle.
- Age: 44
- Gender: Female
- Occupation: Nurse
- Hobbies: Enjoys biking, running and hiking
- Symptoms: Recurrent knee pain for six months
- Prior treatments: ACL reconstruction two years prior with chondroplasty for cartilage lesion on LFC
- Imaging: Revealed full-thickness cartilage defect on LFC with no significant joint space narrowing or malalignment
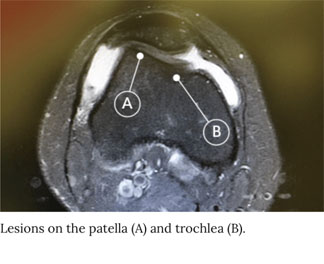 Patient 3 has a 9 cm2 trochlea defect and a 4 cm2 patella defect, both in the right knee.
Patient 3 has a 9 cm2 trochlea defect and a 4 cm2 patella defect, both in the right knee.
- Age: 20
- Gender: Female
- Occupation: Student
- Hobbies: Volleyball player
- Symptoms: Pain, swelling, instability >5 years recurrent injury
- Prior treatment: TTO and lateral release
- Imaging: Revealed defects on the trochlea and patella
Please see below for full indication and ISI.

